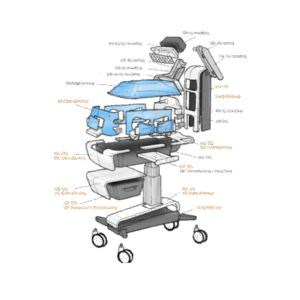
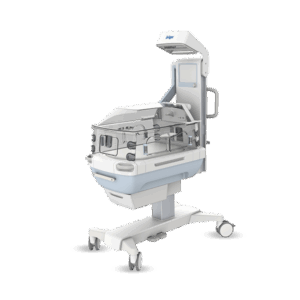
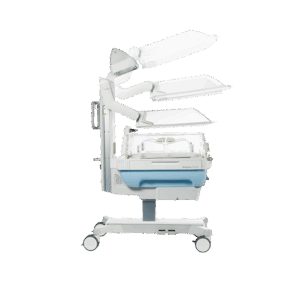
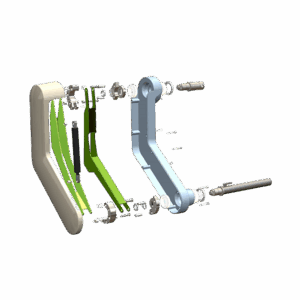
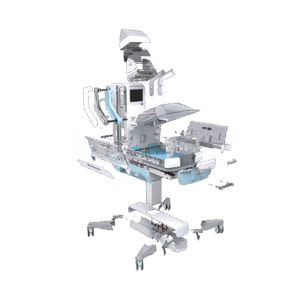
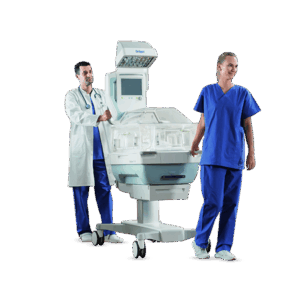
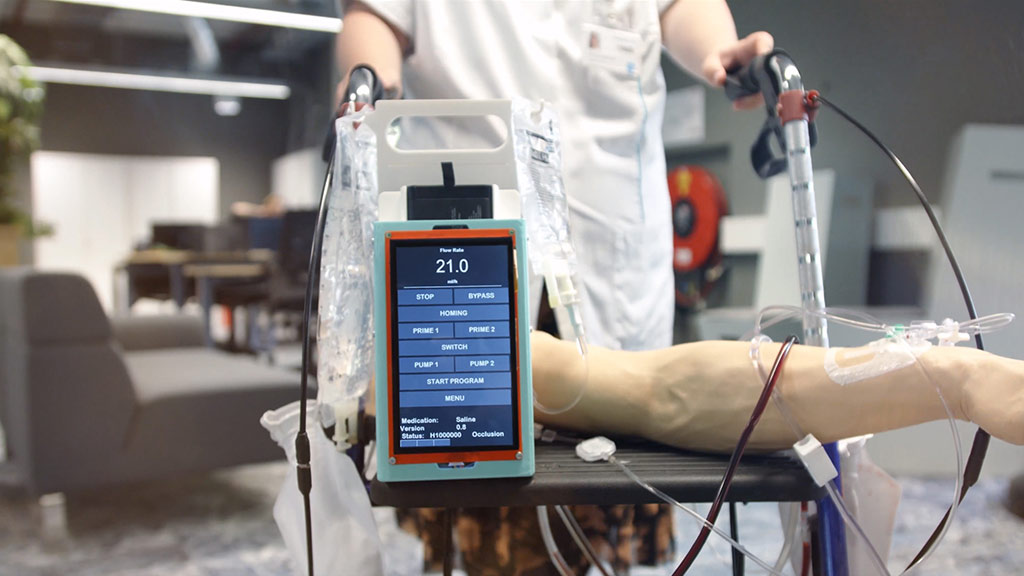
End-to-end product design and development for the healthcare sector
At MMID, we provide medical device development services, from first idea to validated, manufacturable product. Our teams specialize in the design and development of medical and laboratory devices that meet strict regulatory standards while securing user safety, clinical performance, and real-world usability.
Whether you’re building diagnostic equipment, therapeutic systems, or portable care devices, we support you through every phase of medical device product development. With our multidisciplinary teams and ISO 13485 certification, we combine innovation with compliance to deliver trusted solutions for the healthcare sector.
What kind of medical devices do we develop?
From neonatal care to home treatment and preventive health, MMID delivers full-service medical and laboratory product development tailored to each clinical context. Our teams design and engineer solutions that improve patient outcomes, support healthcare professionals, and meet the highest usability and safety standards.
We combine deep technical knowledge with user insights to turn complex challenges into validated, ready-for-production products. Whether it’s for hospitals, labs, or at-home care, we support every stage of end-to-end medical device design, from exploration to certification.
Each project reflects MMID’s commitment to healthcare product innovation , combining engineering, design, and compliance in one integrated process.
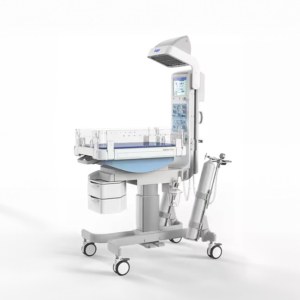
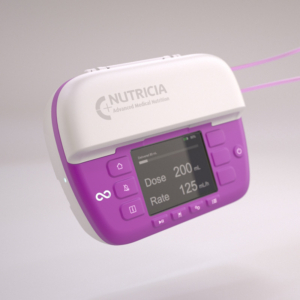
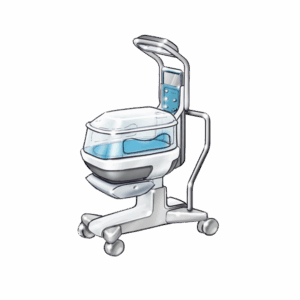
Here are some examples of what we’ve designed and delivered:
How MMID’s healthcare expertise makes the difference
As a medical device development company with over 30 years of experience, MMID helps clients navigate complex projects in a structured, collaborative way. From start-ups to global healthcare brands, we’ve supported the development of user-friendly, production-ready solutions across the healthcare sector.
We’re an ISO 13485 certified medical device design company, experienced in translating clinical requirements into safe, functional, and intuitive devices. Our multidisciplinary teams combine engineering, design, usability, and risk management to ensure your product meets both user needs and regulatory demands.
With a long track record in healthcare and laboratory product development, we offer speed, structure, and the certainty that your product is in expert hands.
Our medical device product development process
Developing medical devices requires more than just creativity, it demands structure, regulations, and deep user understanding. MMID’s medical device development process is built around our certified LUCID method that is compliant to ISO 13485 , guiding clients from idea to validated, market-ready product. Below is how each of the seven stages applies to healthcare:
1. Vision
We explore clinical needs, market trends, and applicable regulations such as FDA Class I/II/III or MDR. We conduct user and stakeholder research, competitor analysis, and align on strategic goals. Our team ensures that the mandatory design input is collected, ensuring compliance with ISO 13485 and relevant healthcare standards.
2. Definition
We generate and visualize multiple design directions, develop functional solutionis, and gather early user feedback. Concept risk assessments are conducted to identify technical or regulatory challenges early in the medical device product development process. Well documented development, requirement management and risk management, secure full grip on the development process.
3. Specification
We define the full specification of the device, including functional, design, production specifications such as: selected components, materials, tolerances, interfaces etc. We prepare verification protocols and ensure that every design decision is traceable, documented, and ready for formal testing.
4. Verification
We develop and test a fully functional and looking like series product prototype against previously defined requirements. This includes usability testing, technical validation, and maintaining complete traceability for design change management.
5. Optimization
Learnings from verification are implemented to refine the product. We improve mechanical robustness, manufacturability, and cost-effectiveness, without compromising compliance or usability.
6. Realisation
Production tooling and small series are finalized. We work with selected suppliers to align manufacturing capabilities with medical-grade requirements and ensure a smooth transfer to production. Quality control on achieving specifications, ensures the safety and effectiveness of the product.
7. Implementation
The medical device is validated for market release. Regulatory documentation is completed, including submission files. Once approved, the product enters the market where MMID can support in evaluating post-market surveillance and product lifecycle follow-up.
Regulatory compliance built into every step
In healthcare product development, there’s no room for shortcuts. At MMID, medical device compliance is embedded into every phase of our process, not as an afterthought, but as a guiding principle.
We’re experienced with major international standards, including ISO 13485, FDA regulations (Class I, II, III), and the EU Medical Device Regulation (MDR). Whether you’re preparing a new diagnostic device, wearable system, or lab solution, we help you meet the necessary regulatory requirements for global markets.
From early concept to final implementation, our teams ensure that your Design History File (DHF) is properly structured and maintained. Risk management, traceability, and documentation are integrated into our workflow. This makes sure that you have all of the input to stay audit-ready and meeting standards throughout the entire medical device product development lifecycle.
With MMID, you gain a development partner who understands not just how to create a great product, but how to ensure it meets the rigorous demands of the healthcare industry.
Case studies: proven success in medical and laboratory innovation
We’ve helped clients bring groundbreaking healthcare products to life. From critical care devices to user-friendly home treatments. Explore how our multidisciplinary teams turn complex healthcare challenges into validated, production-ready solutions.
FAQ about medical device development
What medical devices does MMID develop?
We support the development of a wide range of medical and laboratory devices including diagnostic equipment, infusion systems, wearable health devices, neonatal care solutions, and needle-free injection systems. All products are designed for reliability, usability, and compliance.
How can I bring my complex medical device to market successfully?
Success starts with structure. MMID uses a phased, ISO 13485–certified process that integrates risk management, regulatory strategy, and user validation from the start. We guide you from concept to launch with a clear path forward.
What are the phases of medical device development?
We follow our 7-stage LUCID method: Vision, Definition, Specification, Verification, Optimization, Realisation, and Implementation. Each step is designed to ensure technical quality, user value, and regulatory readiness.
What services do you offer in the medical design process?
Our services include product strategy, concept development, detailed engineering, usability testing, prototyping, supplier coordination, small series production, and documentation support for certification.
What regulations do I need to consider?
Depending on your market, key regulations include FDA (Class I, II, III), EU MDR, and ISO 13485. We help you identify and address applicable requirements throughout the development lifecycle.
How do you ensure user safety and compliance?
We involve real users early and often — testing usability, functionality, and safety. At the same time, we maintain full traceability and support the creation of documentation like DHFs, risk analyses, and verification reports to ensure full medical device compliance.
Bring your medical device to life
Looking for a medical device development company that understands complexity, compliance, and real user needs? Our team of medical device product development consultants is ready to support you — from concept to certified product.
Reach out to discuss your challenge or plan your next step.