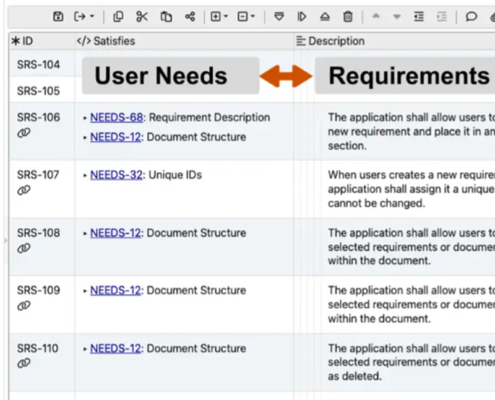
“In the quest to innovate within the medical sector, understanding the intricate dance between regulatory precision, quality management, and user-centric design isn’t just beneficial—it’s imperative for saving lives and enhancing health outcomes.”
The process for developing medical devices is complex. This is especially true because in the medical segment, the stakes are significantly higher when you’re working with patients’ wellbeing, health, and sometimes even their lives. This article outlines medical-specific tips for product development that anyone entering the medical segment must know.